You are here :
- Unité de recherche
- CY LPPI
- Home page
- Research activities
Polymers, Polymer Networks and interpenetrating polymer networks (IPNs)
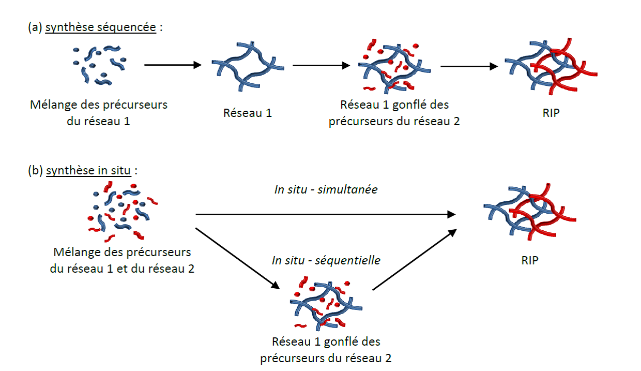
Almost all materials developed correspond to the combination of compounds that are, generally speaking, thermodynamically incompatible. Thus, the architecture of interpenetrating networks of polymers is the only way to develop a mix of two polymers that is stable over time. It corresponds to the combination of crosslinked polymers, at least one of which is synthesized in the presence of the other. These various synthesis pathways are schematized below:
One of the objectives of constituting these architectures is to combine within one material the different properties of each of the associated elements while lessening the weaknesses of each. IPNs are stable dimensionally over time and may even exhibit improved properties of resistance to chemical and physical aging.
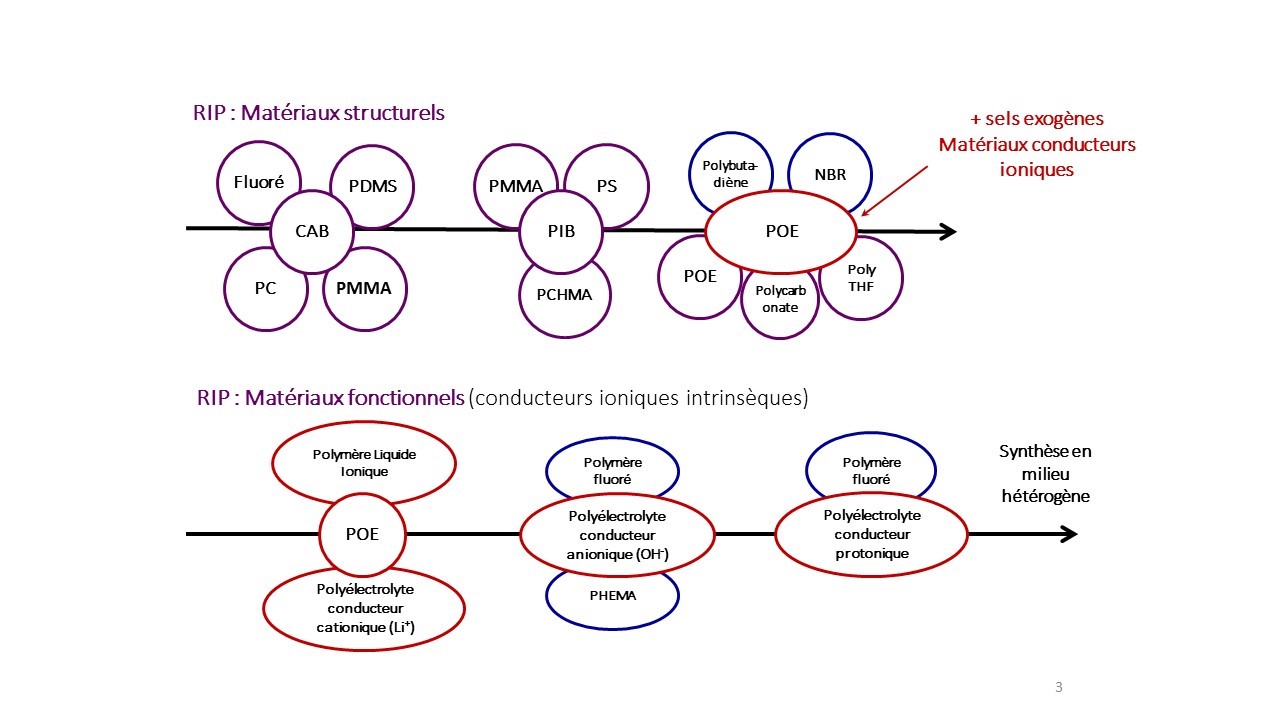
Some associations made:
In order to understand and control the phase separation mechanisms that play such a crucial role in the properties of the final material, the laboratory also produces and investigates structures such as these starting from micro-emulsions and also from 2D systems formed on the air–water interface or developed as a thin film. Under these conditions, the interface phenomena become dominant and therefore easier to characterize. We have thus shown that synthesis at the air-water interface leads to the formation of semi-interpenetrating 2D networks without phase separation, with partners as different as polydimethylsiloxane (PDMS) and cellulose aceto-butyrate. (A. El Haitami et al. 2015, 2014 )
- Interpenetrating Polymer Networks
-
The architecture of interpenetrating polymer networks (IPNs) corresponds to the combination of cross-linked polymers, at least one of which is synthesized in the presence of the other. The different ways of synthesizing IPNs are summarized below:
IPNs represent the only possible mode of association of two cross-linked polymers, that is to say the only way of elaborating a stable “mix” over time of two polymers. If one of the polymers is not cross-linked, we then refer to a semi-interpenetrating polymer network.
One of the objectives of constructing these architectures is to combine within one material the different properties of the associated partners while lessening the weaknesses of each. IPNs are dimensionally stable over time and may even exhibit improved properties of resistance to chemical and physical aging.
- IPN synthesis parameters conditioning the end properties of the material
-
We have shown that the synthesis parameters significantly affect the morphology of end materials.
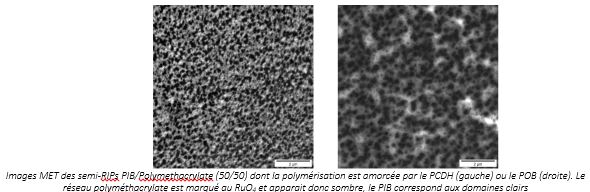
For example, polyisobutene (PIB) with an average molar mass (between 10,000 and 100,000 g/mol) can be immobilized in a polymethacrylate (semi-IPN) network in a solvent-free synthesis. The images below show that the PCHMA-rich phase (dark domains) is in the form of spherical domains from 0.1 to 0.3 µm in diameter, regardless of the initiator of the radical polymerization used. On the other hand, PIB-rich domains (clear domains) are smaller when network formation is initiated by peroxide dicyclohexyl peroxydicarbonate ((PCDH) t½ = 10h at 40°C - low temperature initiator) rather than benzoyl peroxide (POB- t½ = 10h at 80°C).
MET image of semi-IPNs PIB/Polymethacrylate (50/50) whose polymerization is initiated by PCDH (left) or POB (right). The polymethacrylate network is marked with RuO4 and therefore appears dark, while the PIB corresponds to the clear domains.
This difference in morphology was confirmed by dynamic thermomechanical analyses (DMTA). Thus, when the synthesis temperature is higher (POB), the viscosity of the reaction medium decreases, which promotes the separation of phases between polymers during synthesis.
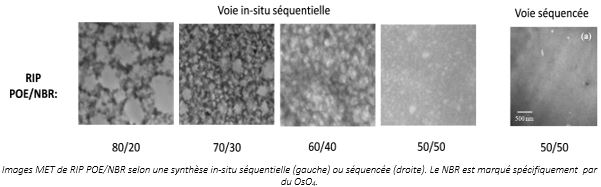
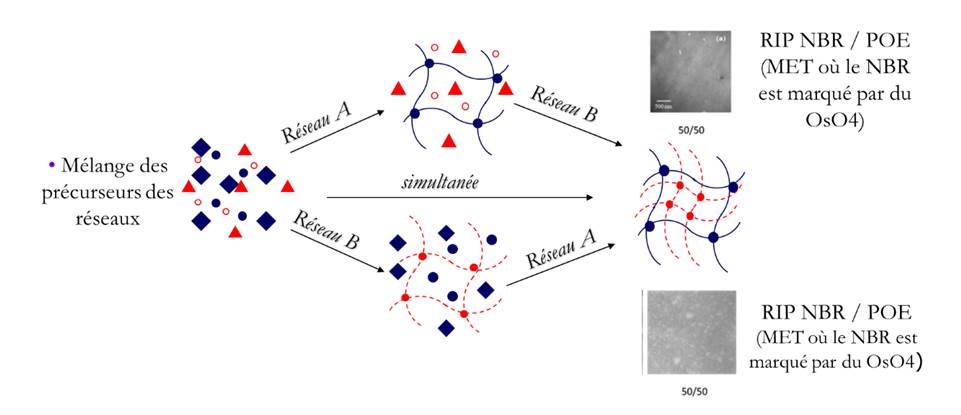
Control of the synthesis parameters is even more important during IPN architecture synthesis. For example, the synthesis of OPE/NBR IPNs was performed sequentially in-situ or sequenced. When the OPE network is formed in the presence of already cross-linked NBRs (sequenced), the phase separation is not detected by MET (figure on right below). However, when the OPE network is formed first (in-situ), it takes the form of microgels whose size increases with the proportion of OPE in the material. The resulting materials then present a macro-separation of phases, leading to a morphology of OPE nodules dispersed in a continuous elastomer phase (figure on left below). As the mass proportion of OPE increases, the OPE domains percolate, totally or partially, leading to a continuous conductive phase.MET images of OPE/NBR IPN according to in-situ sequential (left) or sequenced (right) synthesis. The NBR is specifically marked with OsO4.
- 2D hybrid structures
-
Model 2D structures are formed by adsorption of metal ions under a stabilized fatty acid monolayer at the air-water interface. These structures attempt to mimic those of certain biomaterials such as mother-of-pearl. The metallic ion interaction – the polar organic head appears thin: some divalent cations form a 2D network of ionic complexes commensurable with the organic network (superstructure) while others fail to organize. In order to analyze the relevant physical processes causing growth of a given mineral structure, the adsorption kinetics of different cations were investigated according to different physico-chemical parameters (ion concentrations, pH, counter-ions). Characterizations by thermodynamic measurements, Brewster microscopy and grazing and high-resolution X-ray diffractions (ESRF) have shown that the 2D hybrid structures formed by the Mn2+ and Mg2+ ions exhibit remarkable rigidity and ensure an organization of the organic part not observed on pure water. Study of the adsorption kinetics of Cu2+ ions revealed the role of pH on the phase transitions leading to the final state, which in itself is independent of pH.
- 2D organic structures
-
The methods of making photosensitive surfaces play a significant role in the photochemical processes intervening later. Thus the properties of films of a cellulosic derivative bearing cinnamate groups (CABg) obtained by Langmuir-Blodgett deposit and spin-coating have been compared with those of the corresponding materials synthesized in the form of networks. Photo-irradiation in the UV light of these systems causes the trans→cis isomerisation of the cinnamate photosensitive groups but may also be accompanied by their dimerisation, the importance of which depends on the method of organization of the surface. Organization of the CABg in the form of ultrathin films (Langmuir-Blodgett) or their cross-linking within materials prevents the dimerisation reaction. Conversely, this irreversible reaction is predominant in spin-coating films.
The UV-related surface properties of an azobenzene acrylate and fluorinated acrylate (poly(Azo-co-AcRf6)) copolymer were also investigated. In this case, the isomerisation of the azobenzene groups is only promoted when the film of copolymers is obtained by spin-coating.
The combined study of these different methods of making photosensitive surfaces has therefore made it possible to determine, for each system, the best way of implementing photomodulatable wettability surfaces.